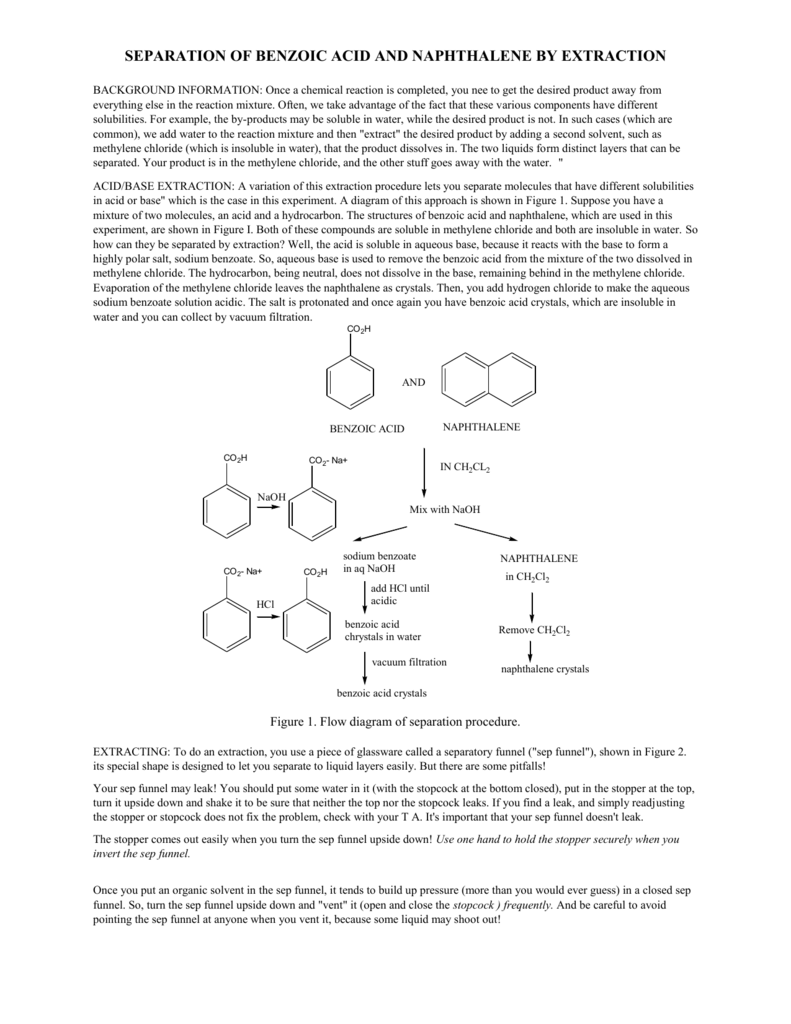
These recommendations are for initiation of therapy. So extracting a mixture of these two compounds with bicarbonate results in the ionization and extraction of a carboxylic acid in the presence of phenol thus.

Propose A Procedure To Separate Aniline From Naphthalene Study Com
Extraction Of Benzoic Acid From Naphthalene

Separating Acids And Neutral Compounds By Solvent Extraction Homeworklib
C OH O NH2 Cl Benzoic acid mp 122-123 4-Chloroaniline mp 68-71 Naphthalene mp 80-82.

Acid base extraction flow chart benzoic acid. Benzoic acid ethyl-4-amino. For non-ionized weak base B B H 2O OH BH Base 1 Acid 2 Base 2 Acid 1 The dissociation constant or basicity constant Kb can be expressed as follows. Thereafter choice of regimens generally rests on.
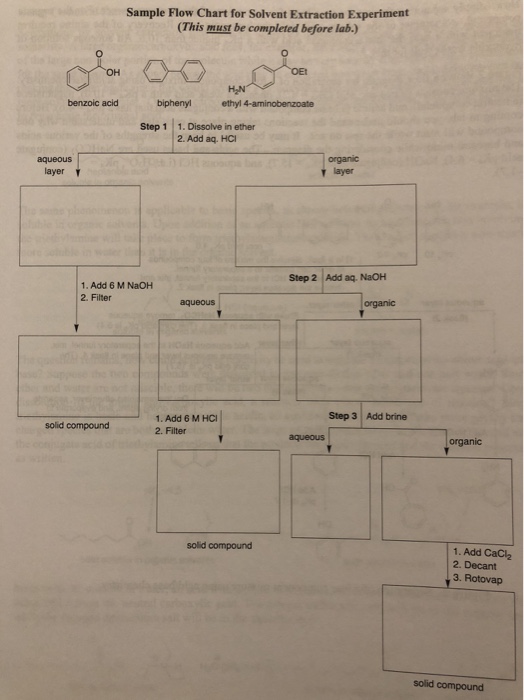
NH4 H2O H 3O NH3 Acid 1 Base 2 Acid 2 Base 1 BPUT MPharm. Prepare a similar flow chart for this experiment. Your flow chart must begin.
Acid-base reactions favor the side with the weaker acid that is they favor the side with the larger pKa. 2016If prediabetes is left untreated 15 to 30 of people with it progress to type 2 diabetes within 5 y American Medical Association and CDC 2015Type 2 diabetes is a major contributor to morbidity mortality and health care costs. Conjugate base of benzoic acid is an ion it is more soluble in water than ether and thus partitions into the water layer.
Canned Food Spoilage Low Acid pH 46 Acid pH 3740 to 46 High Acid pH. ACID BASE Construct a flow chart that follows the separation of a mixture of 2-nitrophenol an organic acid not used in this experiment with a pKa 726 and anthracene neutral organic. Basic principles of work in the analytical laboratory and Classical Methods of analysis Volumetric and Gravimetric Analysis DR.
Mass flow rate Volumetric flow rate density of water 7079 103 m3s 1000 kgm3 7079 kgs EXAMPLE 22 Determine the conversion factor for poundal to newton 03048 m s2 Solution 1 poundal is 1 ft lbs2 1 ft 01383 kg m 01383 Ns2 The conversion factor is 01383 Npoundal. This page discusses the Test Methods for Evaluating Solid Waste. Basic Extraction of an Organic Acid At this point you can separate the organic ether layer and the aqueous layer.
An acid-base extraction operates on the same principle but can provide a further level of fine -tuning. OH O NH 2 N O-O 3 4-nitroaniline. If one or more of the compounds in the mixture to be separated is acidic or basic.
Such physiologic regimens allow greater freedom of lifestyle because patients can skip or time-shift meals and maintain normoglycemia. The neutral N-4-nitrophenylbenzamide will remain in the diethyl ether. For flow chart use N for neutral -RCO 2H for protonated carboxylic acid RCO 2 for ionic carboxylate salt RNH 2 for neutral amine and RNH 3 for ionic ammonium salt.
Benzoic acid will be extracted with an aqueous base while 4-nitroaniline will be extracted with an aqueous acid. You dont need to track down any data on the indicator in part 1 everything you need is in this handout. 3 Calculate the number of mmol thats milimoles of benzoic acid benzocaine.
Prediabetes is associated with an increased risk of cardiovascular disease coronary heart disease stroke and all-cause mortality Huang et al. They provide the theory and key practical aspects of flow cytometry enabling immunologists to avoid the common errors that often. The pKa of the conjugate acid of benzocaine is 25.
EXAMPLE 23 Express kgfcm2 and lbfin2 as Nm2. Figure 2 Figure 2. Write balanced chemical equations for the reactions of a sodium hydroxide with benzoic acid b hydrochloric acid with sodium hydroxide and c hydrochloric acid with.
These guidelines are a consensus work of a considerable number of members of the immunology and flow cytometry community. Then if the basicity of acid is n molecular weight of acid would be w2 1 Msalt w1 and molecular weight of acid M n107 salt 108 n This is one good practical application of POAC. Your flow -chart must use benzoic acid and naphthalene.
For each extraction place a plastic sample in a clean 25 millimeters 200 millimeters hard-glass test tube and add solvent equal to 10 milliliters of solvent per square inch of plastic surface. 15th May 2019 400-500 pm. In the Extraction experiment liquid-liquid extraction was used to separate and purify naphthalene and benzoic acid from a mixture of the two through a separatory funnel.
This amount will be between 45 milliliters and 55 milliliters. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solventThe solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature pressure and presence of other chemicals including changes to the pH of the solution. The MATERIALS database contains chemical physical visual and analytical information on over 10000 historic and contemporary materials used in the production and conservation of artistic architectural archaeological and anthropological materials.
This is clearly wrong as an acid solution should have a pH of less than 7. Sem-1 Chapter-1 Preformulation Studies A. Samanta 3 H 3 O NH 3 In this case the acidity constant Ka NH 4 Ionization of weak bases Case1.
Subjected to the indicated extraction conditions. The pKa of benzoic acid is 42. The flow chart on the next page outlines a general procedure for separating acidic basic and neutral organic compounds using the principles of the solubility switch.
Be sure to include appropriate structures of the compounds and explicitly state what chemicals are present at each step. Treating the system as a mixture of hydrochloric acid and the amphoteric substance water a pH of 689 results. PhysicalChemical Methods compendium or SW-846 which is the EPAs official collection of methods for use in complying with the Resource Conservation and Recovery Act RCRA regulations.
2 shows a flow chart for the separation of benzoic acid 4-nitroaniline and N-4-nitrophenylbenzamide. Separation of a Neutral from a Carboxylic Acid. Extraction Hayley Williams willi553gostocktonedu CHEM 2125 007 February 27 2018 Abstract.
You will separate a mixture that contains benzoic acid 4-chloroaniline and naphthalene. A weak acid or the conjugate acid of a weak base can be treated using the same formalism. The aqueous layer can then be acidified and subsequently extracted with ether to obtain.
Weak acids and bases. As the organic species and. Attach your small metal ring to one of the vertical rods on your rack 2.
The idea behind an acid-base extraction is to utilize the acid-base properties of. Before permanent installation test the equipment with the chemicals and under the specific conditions of your application. The MAUDE database houses medical device reports submitted to the FDA by mandatory reporters 1 manufacturers importers and device user facilities and voluntary reporters such as health care professionals patients and consumers.
The information in this chart has been supplied to Cole-Parmer by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility.
1

Draw A Flowchart That Shows How You Would Seperate Benzoic Acid From 3 4 Dibromophenol Remember Both Compounds Are Acidic Look At Pka Values To Determine Which Is The Most Acidic Study Com
Solved Sample Flow Chart For Solvent Extraction Experiment Chegg Com

How Could You Quantitatively Separate P Ethyl Benzoic Acid From Ethyl Benzoate And Recover Each Material In Pure Form Study Com

Postlab Exp 4 Payal Patel Experiment 5 Extraction Post Lab Results Discussion Solution Of Benzoic Acid Benzocaine And Fluorenone In Methylene Course Hero

Separation Of A Three Component Mixture By Extraction Lab Report Help The Compounds Are Benzoic Acid 9 Fluorene And Ethyl 4 Aminobenzoate I Need Help To Write Discussion About This Lab Report A
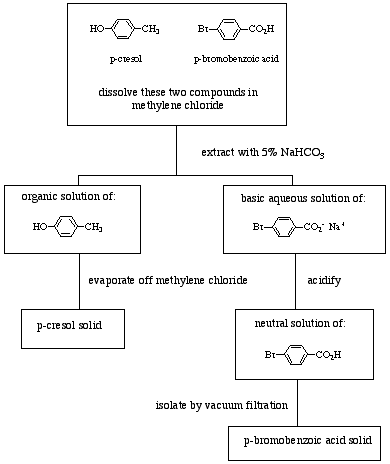
Extraction Technique Quiz

Prepare A Flow Chart For The Separation Of A Three Component Mixture That Contains 4 Chlorobenzoic Acid 3 Nitroaniline And 1 3 5 Trimethylbenzene You Must Show The Structural Formula For Each Compo Study Com